Events
For more information, please contact cs@ewcdiagnostics.com
Heralding Education on Antimicrobial Resistance
De data voor de HEAR-najaarscursus van Liofilchem zijn bekend.
Wanneer: Donderdag 3 oktober– vrijdag 4 oktober 2024
Locatie: Liofilchem, Via Uruguay, 64026 Roseto degli Abruzzi TE, Italië
HEAR courses comprise an interactive two day session with lectures and laboratory work. An international faculty interacts with 20 participants from several Countries. HEAR allows for effective exchange of clinical experiences and expertise among clinicians, pharmacists and microbiologists with the patient’s need utmost in mind. Liofilchem organizes several courses per year that cover a variety of topics with the goal of sharing the latest information to promote the rational use of antimicrobial agents.
Archives

Older News Items
ComASP® Colistin
ComASP® Colistin Downloads Download the product IFU View the complete range of broth microdilution panels
Steel coupons
Steel coupons for hydrogen peroxide sterilization Downloads Download the product IFU View the complete range of Biological
Cursus Levensmiddelenmicrobiologie & -hygiëne
Cursus Levensmiddelenmicrobiologie & -hygiëne In 5 dagen komt een breed scala aan onderwerpen aan de orde. Je leert wat micro-organismen
Mythen, Missers en Maatwerk + Meesterwerk Infectieuze Bedreigingen 2024
Mythen, Missers en Maatwerk + Meesterwerk Infectieuze Bedreigingen 2024 Schrijf je in voor het congres Mythen, Missers en Maatwerk + Meesterwerk
Quanti-CultiControl
Quanti-CultiControl for growth promotion testing of culture media Downloads Download the product technical sheet Download the photographic test procedure
ESC Swab
ESC Swab Downloads Learn more about ESC Swab ESC (Easy Surface Checking) swab
MTS™ Synergy Application System
MTS™ Synergy Application System for in-vitro combination of antibiotics Downloads Learn more about MTS™ SAS Learn more about our
Dehydrated Culture Media
Dehydrated Culture Media A wide range of media, in compliance with ISO 11133 and other international standards Downloads
Iron Sulphite Agar
Iron Sulphite Agar acc. to ISO 15213-1 Downloads Learn more in the product technical sheet. View our
Culticontrol
Culticontrol CultiControl microorganisms are lyophilized, reference stock culture preparations containing a single strain of a microorganism.
Gamma-irradiated contact plates in blister pack
Gamma-irradiated contact plates in blister pack The peelable blister pack fulfills the highest quality and protection
Buffered NaCl Peptone Solution pH 7.0
Buffered NaCl Peptone Solution pH 7.0 Diluent for detection and enumeration of microorganisms according to USP/EP/JP and ISO 21149
Cefepime-enmetazobactam MTS™
new Cefepime-enmetazobactam MTS™ Downloads See the whole range of MTS™ Solutions for the antimicrobial resistance management
Contact Slide
Contact Slide A ready-to-use device with two different media coated onto a plastic carrier used for the microbial monitoring of surfaces
Steam Control GST E6 SS (BA coupons)
Steam Control GST E6 SS (BA coupons) Downloads Download the product technical sheet View the whole Biological Indicators
m-CP Agar
m-CP Agar Selective medium for detection of Clostridium perfringens in water samples Downloads Download the product IFU Learn more
ESC Swab
ESC Swab Downloads Learn more about ESC Swab ESC (Easy Surface Checking) swab
Ready-to-use Agar Dilution panels
Ready-to-use Agar Dilution panels Downloads AD Fosfomycin 0.25-256 Blood AD Clindamycin 0.03-32 Request your custom version!
Aztreonam-avibactam MTS™
Aztreonam-avibactam MTS™ Downloads Download the product IFU MTS™ (MIC Test Strip) whole product range
Custom Broth MicroDilution panels
Custom Broth MicroDilution panels Downloads Click here to learn more about ComASP. Liofilchem

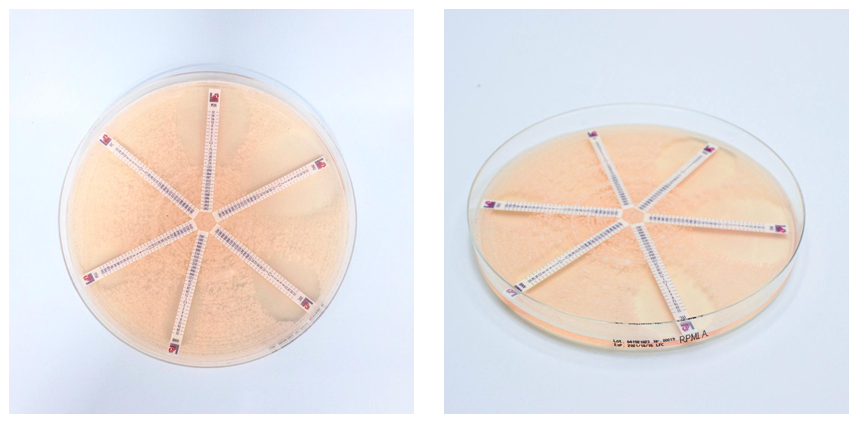
RPMI Agar (RPMI1640, MOPS, L-glutamine, 2% Glucose) is intended for the AFST (antifungal susceptibility testing) and is recommended for usage with Antifungal MTS™ (MIC Test Strip).
Learn more about MTS™ for yeasts
Learn more about MTS™ for moulds
Pick 4-5 colonies from 24-hours old cultures and suspend in saline solution or sterile water in order to obtain a 0.5 McFarland cell density. Introduce a sterile swab into the suspension and inoculate the RPMI Agar plate. Allow the plate to dry, position the antifungal strip onto the agar surface with the maximum concentration nearest the periphery of the plate.
Incubate at 35 °C for 24-48 hours.
Read the M.I.C. value where the edge of the inhibition ellipse intersects the strip (intersection between two scale segments should be round up to the higher value).
Available ready-to-use formats:
Ref. 11509
90 mm agar plate
20/pack
Ref. 10233
140 mm agar plate
10/pack
10-25°C storage temperature
180 days shelf life
EnteroPluri-Test is a 12-sector system containing special culture media for the identification of Enterobacteriaceae and other Gram-negative, oxidase negative bacteria. The system allows the simultaneous inoculation of all media present in the sectors and 15 biochemical reactions. The microorganism is identified by evaluating the color change of each culture medium after 18-24 hours of incubation at 36±1°C and by a code number obtained from the biochemical reaction interpretation.
Ref. 78618 – 10/pack
Ref. 78619 – 25/pack
Storage temperature: 2-8°C
Shelf life: 240 days

Liofilchem confirms its continuous dedication to meet the pharmaceutical and cosmetics microbiology needs with the introduction of TAT Broth, a ready-to-use liquid medium for the microbiological examination of cosmetics and topical drugs, particularly from highly viscous or gelatinous materials. This medium, also known as Fluid Casein Digest – Soy Lecithin – Polysorbate 20 Medium (SCDLP 20 Broth), complies with ISO 21149 for detection of aerobic mesophilic bacteria.
Liofilchem also produces Eugon LT SUP, a highly nutritious medium used for the detection and enumeration of microorganisms in cosmetic products and TSB + Capitol 4, particularly suitable for testing viscous samples.
Antimicrobial resistance management
new Chromatic Super CAZ/AVI
Chromogenic medium for the detection of Ceftazidime-avibactam resistant Gram-negative bacteria
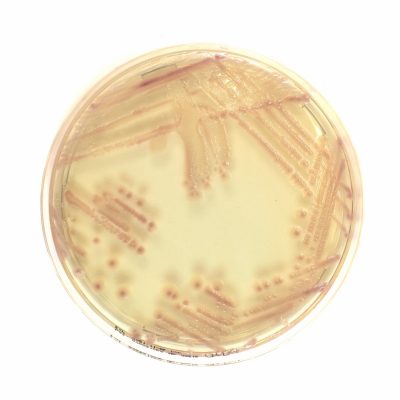
Chromatic Super CAZ/AVI is a selective and differential chromogenic medium used for screening ceftazidime-avibactam resistance in Enterobacterales and Pseudomonas aeruginosa.
Results can be interpreted after incubation for 18-24 hours. Subculture to a non-selective medium is required for confirming identification, antimicrobial susceptibility testing and epidemiological typing.
Ref. 11641
90 mm plates, 20/pack
2-8°C storage temperature
4 months shelf life
VTM
Transport medium for Viruses, Chlamydia, Mycoplasma and Ureaplasma
VTM is suitable for collection and preservation of clinical specimens containing viruses, including SARS-CoV-2 (COVID-19), Chlamydia spp., Mycoplasma spp. and Ureaplasma spp. The transport medium can be used with swabs and aspirates and is available in a plastic, screw cap tube (or a bulk format, 200 mL glass bottle). The transported microorganism remains viable for 48 hours at room or refrigerated temperature. VTM does not contain guanidium thiocyanate.
Features:
• 10-25°C storage temperature.
• 1 year shelf life.
• Contains antimicrobial agents to minimize bacterial and fungal contamination, without affecting viruses, Chlamydia, Mycoplasma or Ureaplasma.
• A pH indicator (red phenol) indicates the medium integrity during the shelf life of the product.
• The tube stands upright on the laboratory bench thanks to the skirted, flat-bottom, while the conical shape allows an appropriate centrifugation of the sample.
• 16x100mm skirted tube with plastic red cap. Sterile. 100/pack

Introducing Accu-Bac
TCS is excited to announce our innovative new quantitative micro-organism product, Accu-Bac.

Accu-Bac is a guaranteed second generation derivative of NCTC cultures, manufactured under licence from Public Health England. Each carefully prepared disc will contain a target range of ?100 c.f.u, with 95% confidence interval stated on the Certificate of Analysis.
This is ideal for most quantitative applications. Accu-Bac will be supplied in 1 disc vials and has a 12 month shelf-life from the date of manufacture.
- Target c.f.u. counts of ?100 per disc, with 95% confidence interval range stated on the Certificate of Analysis
- Shelf life of 12 months
- Simple to use, no dilutions required
- Guaranteed 2nd generation and fully traceable to the original NCTC source strain
- Certificates of Analysis available from our website
- Identification and Characterisation testing completed in accordance with our UKAS accredited scope
| Ref | Description | Strain Designation |
| AB17 | Enterococcus faecalis | NCTC 775 / ATCC® 19433 / WDCM00009 |
| AB75 | Escherichia coli | NCTC 9001 / ATCC® 11775 / WDCM00090 |
| AB26 | Enterobacter aerogenes | NCTC 10006 / ATCC® 13048 / WDCM00175 |
| AB40 | Pseudomonas aeruginosa | NCTC 12924 / ATCC® 9027 / WDCM00026 |
| AB04 | Klebsiella pneumoniae | NCTC 9633 / ATCC® 13883 / WDCM00097 |
Liofilchem®
AF Genital System
Liofilchem®
AF Genital System
A.F. Genital System (ref. 74156) is a 24-well panel containing biochemical substrata and antimicrobial drugs for detection, presumptive identification and susceptibility testing of microorganisms directly from urogenital specimens (vaginal swab, urethral swab, seminal fluid, urine).
The new improved configuration allows to separately identify and enumerate Ureaplasma spp. and Mycoplasma hominis.
Count of Ureaplasma spp.(Uu) 10e3 to ? 10e5 CFU/mL
Count of Mycoplasma hominis (Mh) 10e4 to ? 10e5 CFU/mL
Bacterial and fungal screening:
Escherichia coli
Proteus spp. / Providencia spp.
Pseudomonas spp.
Gardnerella vaginalis
Staphylococcus aureus
Enterococcus faecalis
Neisseria gonorrhoeae
Streptococcus agalactiae (Group B)
Candida spp.
Just like all the Liofilchem direct microbial ID systems, the AF Genital System has the great benefits of:
- performing simultaneous microbiological screening of several microorganisms in one compact panel.
- reducing costs in man hours and laboratory consumables.
- easy to use and no equipments required.
Liofilchem®
Antimicrobial resistance management
Liofilchem®
Antimicrobial resistance management
Isavuconazole MTS™
  |
|---|
Isavuconazole MTS™ (MIC Test Strip), IVU 0.002-32 ?g/mL , is a quantitative method for the in-vitro susceptibility testing of Candida spp., Aspergillus spp. and Mucorales.
Isavuconazole is a novel broad-spectrum triazole agent with activity against a variety of opportunistic and pathogenic yeasts and moulds. Isavuconazole can be administered either orally or intravenously as a water soluble prodrug, isavuconazonium sulfate (Cresemba®), which then is rapidly cleaved by esterases into active component and an inactive prodrug cleavage product. Isavuconazole inhibits ergosterol synthesis in the fungal membrane. Previous in vitro studies have shown good activity against all Candida spp and species of Aspergillus and Mucorales. Potent activity against Cryptococcus spp and other emerging yeast infections has been demonstrated as well.
Isavuconazole MTS™ 0.002-32 ?g/mL
ref. 921841 – 10/pack (individually packed)
ref. 92184 – 30/pack (individually packed)
ref. 921840 – 100/pack

HIMEDIA
Leading BioSciences Company
Mucormycosis detection
HIMEDIA
Leading BioSciences Company

We want to draw your attention to a newly developed product (range).
As you have probably heard some of the COVID infected patients suffer in addition to a super-infection of moulds.
To be able to detect this HiMedia has developed the necessary detection material.
- K144 => Mucormycosis Detection Kit
- MP5477 => Candida Selective Agar Plate
- MS5478 => HiFungalTM Transport Medium w/ swabs
- MP5476 => Mucormycosis Selective Agar Plate
- And the brochure about Mucormycosis
The prices will follow asap. The brochure and leaflets can be found HERE.
Liofilchem®
D-value verification
Liofilchem®
D-value verification
Liofilchem performs D-Value and Z-Value analysis by direct inoculation of product or equipment using Biological Indicator Organisms (BI’s).
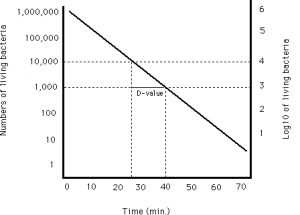
D-values are stated by the manufacturer on the Biological indicator but often require verification for a higher degree of accuracy and process specificity. The Pharmacopoeias and ISO11138 stress on the importance of D-value verification to ensure the accuracy of a stated manufacturer D-value.
Our Biological Indicator Evaluation Resistometer (BIER) Vessel autoclave verifies the D-value by accurately delivering a calibrated and pre-determined level of lethality.
The log reduction achieved for exposure time is assessed through the exposure of the BI for defined time points and interpretation via fractional analysis. This process allows the accurate verification of the manufacturer’s stated D-value.
Learn more about our D-Value and Z-Value determination protocols
Liofilchem®
Tryptic Soy Agar + Neutralizing
Settle plate for air monitoring
Liofilchem®
Tryptic Soy Agar + Neutralizing
Settle plate for air monitoring
The 90 mm settle plates contain 30 mL of agar to compensate for desiccation after active sampling of 1000 liters of air and are suitable for prolonged incubation to favor the detection of microorganisms.
Gamma-irradiated, triple envelope
- 30 mL agar plate
- 20 plates/pack
- Catalog ref. 10059S
- 10-25°C storage temperature
- 6 months shelf life
- 2D data matrix code on each plate
Liofilchem®
Microbiology products
new Cefiderocol MTS™
Liofilchem®
Microbiology products
Cefiderocol MTS™ (MIC Test Strip), FDC 0.016-256 ?g/mL , is a quantitative method for the in-vitro susceptibility testing of Pseudomonas aeruginosa to Cefiderocol.
MTS™ consists of special porous paper impregnated with a pre-defined concentration gradient of an antimicrobial agent, used to determine the minimum inhibitory concentration (MIC) in ?g/mL of antimicrobial agents against bacteria as tested on agar media using overnight incubation and manual reading procedures.
Cefiderocol is a siderophore cephalosporin with activity against aerobic Gram-negative organisms. It binds to free iron and is actively transported into bacterial cells through the outer membrane. This Trojan horse strategy allows cefiderocol to enter the space in-between the bacterial cell walls and disrupt cell wall synthesis. In addition, cefiderocol is stable against nearly all beta-lactamases, including both the serine and metallo-carbapenemases.
Cefiderocol MTS™ 0.016-256 ?g/mL
ref. 920671 – 10/pack (individually packed)
ref. 92067 – 30/pack (individually packed)
ref. 920670 – 100/pack
CultiControl
Freeze-dried microorganisms
CultiControl
Freeze-dried microorganisms
|
 |
 |
|---|---|
| The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark and the ATCC catalog marks are trademarks of ATCC. Liofilchem is licensed to use these trademarks and to sell products derived from ATCC® cultures. | NCTC® is a registered trademark of Public Health England. Liofilchem is licensed to use these trademarks and to sell products derived from NCTC® cultures. |
Chromatic Bacillus cereus
Chromatic Bacillus cereus
|
Hamilton, MolGen, and EWC Diagnostics join forces to scale up SARS-CoV-2 testing capacity in the Netherlands
Today, Hamilton, MolGen, and EWC Diagnostics announce their strategic partnership to scale up SARS-CoV-2 testing capacity in the Netherlands.
Since the outbreak of the COVID-19 pandemic in Europe, demand for SARS-CoV-2 detection tests has significantly increased. In order to increase testing capacity, the Dutch government, through the Ministry of Health, Welfare and Sport, invited commercial organizations to respond to a tender released this summer.
PathoFinder and EWC Diagnostics partnership to meet COVID-19 testing demands in the Netherlands.
PathoFinder and EWC Diagnostics announce their collaboration to rapidly advance the expansion of COVID-19 testing capacity in the Netherlands by providing the Dutch government with complete sets of sampling materials and SARS-CoV-2 PCR kits.